Geometric and electronic structure of Pd/4-aminothiophenol/Au(111) metal–molecule–metal contacts: a periodic DFT study
Abstract
Periodic density functional theory calculations were performed to address the geometric and electronic structure of a
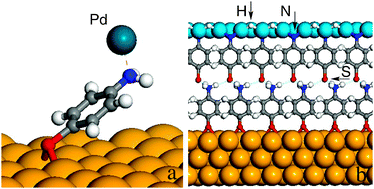
- This article is part of the themed collection: Interfacial Systems Chemistry: Out of the Vacuum, Through the Liquid, Into the cell

 Please wait while we load your content...
Please wait while we load your content...