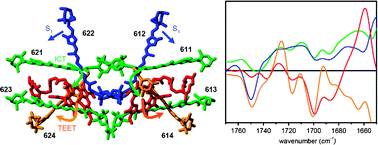
The peridinin chlorophyll-a protein (PCP) is a water–soluble, trimeric light harvesting complex found in marine dinoflagellates that binds peridinin and Chl-a in an unusual stoichiometric ratio of 4 : 1. In this paper, the pathways of excited-state energy transfer and relaxation in PCP were identified by means of femtosecond visible-pump, mid-infrared probe spectroscopy. In addition, excited-state relaxation of peridinin dissolved in organic solvent (CHCl3 and MeOH) was investigated. For peridinin in solution, the transient IR signatures of the low-lying S1 and intramolecular charge transfer (ICT) states were similar, in line with a previous ultrafast IR study. In PCP, excitation of the optically allowed S2 state of peridinin results in ultrafast energy transfer to Chl-a, in competition with internal conversion to low-lying optically forbidden states of peridinin. After vibrational relaxation of the peridinin hot S1 state in 150 fs, two separate low-lying peridinin singlet excited states are distinguished, assigned to an ICT state and to a slowly transferring, vibrationally relaxed S1 state. These states exhibit different lactone bleaches, indicating that the ICT and S1 states localize on distinct peridinins. Energy transfer from the peridinin ICT state to Chl-a constitutes the dominant energy transfer channel and occurs with a time constant of 2 ps. The peridinin S1 state mainly decays to the ground state through internal conversion, in competition with slow energy transfer to Chl-a. The singlet excited state of Chl-a undergoes intersystem crossing (ISC) to the triplet state on the nanosecond timescale, followed by rapid triplet excitation energy transfer (TEET) from Chl-a to peridinin, whereby no Chl-a triplet is observed but rather a direct rise of the peridinin triplet. The latter contains some Chl-a features due to excitonic coupling of the pigments. The peridinin triplet state shows a lactone bleach mode at 1748 cm−1, while that of the peridinin ICT state is located at 1745 cm−1, indicating that the main channels of singlet and triplet energy transfer in PCP proceed through distinct peridinins. Our results are consistent with an energy transfer scheme where the ICT state mainly localizes on Per621/611 and Per623/613, the S1 state on Per622/612 and the triplet state on Per624/614.

 Please wait while we load your content...
Please wait while we load your content...