A Strecker-type synthesis of glycine by reacting NH3, H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and HCN in presence of ice water (H2O–ice) as a catalyst has been theoretically studied at B3LYP/6-31+G(d,p) level within a cluster approach in order to mimic reactions occurring in the interstellar and circumstellar medium (ICM). Results indicate that, despite the exoergonic character of the considered reactions occurring at the H2O–ice surface, the kinetics are slow due to relatively high electronic energy barriers (ΔU≠0 = 15–45 kcal mol−1). Reactions occurring within H2O–ice cavities, in which ice bulk effects have been modeled by assuming a dielectric continuum (ε = 78), show energy barriers low enough to allow NH2CH2OH formation but not NH
O and HCN in presence of ice water (H2O–ice) as a catalyst has been theoretically studied at B3LYP/6-31+G(d,p) level within a cluster approach in order to mimic reactions occurring in the interstellar and circumstellar medium (ICM). Results indicate that, despite the exoergonic character of the considered reactions occurring at the H2O–ice surface, the kinetics are slow due to relatively high electronic energy barriers (ΔU≠0 = 15–45 kcal mol−1). Reactions occurring within H2O–ice cavities, in which ice bulk effects have been modeled by assuming a dielectric continuum (ε = 78), show energy barriers low enough to allow NH2CH2OH formation but not NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (ΔU≠0 = 2 and 21 kcal mol−1, respectively) thus hindering the NH2CH2CN formation, i.e. the precursor of glycine, through Strecker channels. Moreover, hydrolysis of NH2CH2CN to give glycine is characterized by high electronic energy barriers (ΔU≠0 = 27–34 kcal mol−1) and cannot readily occur at cryogenic temperatures. Nevertheless, the facts that NH
CH2 (ΔU≠0 = 2 and 21 kcal mol−1, respectively) thus hindering the NH2CH2CN formation, i.e. the precursor of glycine, through Strecker channels. Moreover, hydrolysis of NH2CH2CN to give glycine is characterized by high electronic energy barriers (ΔU≠0 = 27–34 kcal mol−1) and cannot readily occur at cryogenic temperatures. Nevertheless, the facts that NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 formation can readily be achieved through the radical–radical HCN + 2H → NH
CH2 formation can readily be achieved through the radical–radical HCN + 2H → NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 reaction [D. E. Woon, Astrophys. J., 2002, 571, L177–L180], and that present results indicate that the Strecker step of NH
CH2 reaction [D. E. Woon, Astrophys. J., 2002, 571, L177–L180], and that present results indicate that the Strecker step of NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 + HCN → NH2CH2CN exhibits a relative low energy barrier (ΔU≠0 = 8–9 kcal mol−1), suggest that a combination of these two mechanisms allows for the formation of NH2CH2CN in the ICM. These results strengthen the thesis that NH2CH2CN could have been formed and protected by icy dust particles, and then delivered through micro-bombardments onto the early Earth, leading to glycine formation upon contact with the primordial ocean.
CH2 + HCN → NH2CH2CN exhibits a relative low energy barrier (ΔU≠0 = 8–9 kcal mol−1), suggest that a combination of these two mechanisms allows for the formation of NH2CH2CN in the ICM. These results strengthen the thesis that NH2CH2CN could have been formed and protected by icy dust particles, and then delivered through micro-bombardments onto the early Earth, leading to glycine formation upon contact with the primordial ocean.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and
O and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (ΔU≠0 = 2 and 21 kcal mol−1, respectively) thus hindering the NH2CH2CN formation, i.e. the precursor of
CH2 (ΔU≠0 = 2 and 21 kcal mol−1, respectively) thus hindering the NH2CH2CN formation, i.e. the precursor of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 formation can readily be achieved through the radical–radical
CH2 formation can readily be achieved through the radical–radical ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 reaction [D. E. Woon, Astrophys. J., 2002, 571, L177–L180], and that present results indicate that the Strecker step of NH
CH2 reaction [D. E. Woon, Astrophys. J., 2002, 571, L177–L180], and that present results indicate that the Strecker step of NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 +
CH2 + 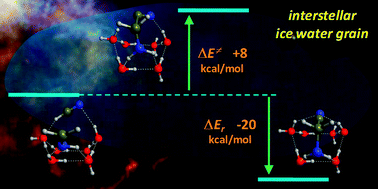

 Please wait while we load your content...
Please wait while we load your content...