Spontaneous product segregation from reactions in ionic liquids: application in Pd-catalyzed aliphatic alcohol oxidation†
Abstract
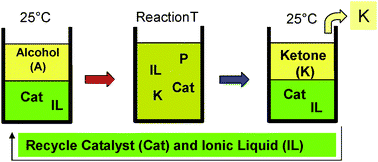
A methodology is introduced to separate polar reaction products from ionic liquids without the need for organic
- This article is part of the themed collection: Ionic Liquids

 Please wait while we load your content...
Please wait while we load your content...