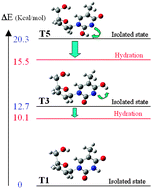
A comparative theoretical conformational analysis of the tautomerism of the four most stable conformers of the antiviral analogue D4T (stavudine) and natural thymidine (Thy) nucleosides was carried out, by using the B3LYP and MP2 quantum chemical methods. The calculated structure data and energy values of the tautomers were compared with the T1 keto form and with the results determined for the thymine molecule. For each conformer, only two stable enol forms, T3 and T5, were obtained when considering different positions of hydrogen around the base. Tautomer T3 always appears more stable than T5, and more stable in the natural nucleoside Thy than in thymine or in D4T.
The effect of water on the tautomers was estimated by using an explicit number of up to five water molecules to surround the nucleoside, and also by Tomasi’s polarized continuum model (PCM). A total of about 200 clusters were optimised and the geometrical parameters and energies discussed. The water net differs in the three tautomers of D4T and Thy. Depending on the nature of the tautomers, cyclic, distributed water molecules, or clustered structures are formed. The deformation and interaction counterpoise (CP)-corrected energies between the nucleoside and water molecules were determined. The relative stabilities of all tautomers were established. The microhydrated environment stabilizes remarkably the enol forms more than the canonical keto one, although this one continues being the most stable. The changes in the intramolecular H-bondings and in the total atomic charges were discussed. Intramolecular H-bonds being stronger in D4T than in Thy could indicate a higher molecular flexibility for Thy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...