The structures of the series of N,N′-1,n-phenylenebis(oxamic acid ethyl ester) molecules with n = 2 (H2Et2opba, 1), 3 (H2Et2mpba, 2), and 4 (H2Et2ppba, 3) have been determined by single-crystal X-ray diffraction (XRD) methods. Density functional (DF) calculations have been performed on the simplest model system N-phenyloxamic acid methyl ester (HMepma). Compounds 1–3 have either folded (H2Et2opba), bent (H2Et2mpba), or linear (H2Et2ppba) almost planar (periplanar) molecular configurations with the two oxalamide moieties being slightly tilted up and down, respectively, with respect to the benzene ring. The energy calculations as a function of the torsion angle (ϕ) around the N(amide)–C(benzene) bond for HMepma reveal that the minimum energy syn and anti periplanar conformations of the carboxamide functions are more stable than the corresponding syn and anti planar ones (ϕ = 0 and 180°) by 0.18 and 0.13 kcal mol−1, respectively. The calculated ϕ values for the syn and anti periplanar minimized conformers of HMepma are 16.0 and 200.0°, respectively, in reasonable agreement with the experimental values for 1–3 [ϕ = 39.0(4) and 225.0(3) (H2Et2opba), 32.6(5) (H2Et2mpba), and 34.7(2)° (H2Et2ppba)]. This situation likely minimizes the forced repulsive interactions between the amide hydrogen and the nearest benzene hydrogen atoms while it maximizes the attractive interactions between the carbonyl amide oxygen and the nearest benzene hydrogen atoms, which are then implicated in a relatively weak, intramolecular C–H(benzene)⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(amide) hydrogen bond [d(H⋯O) = 2.45(2)–2.57(2) Å]. A supramolecular aggregation of molecules into either a duplex (H2Et2opba) or a brick-wall sheet (H2Et2ppba) occurs for 1 and 3, respectively, through moderately strong, intermolecular N–H(amide)⋯O
C(amide) hydrogen bond [d(H⋯O) = 2.45(2)–2.57(2) Å]. A supramolecular aggregation of molecules into either a duplex (H2Et2opba) or a brick-wall sheet (H2Et2ppba) occurs for 1 and 3, respectively, through moderately strong, intermolecular N–H(amide)⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(amide) hydrogen bonds [d′(H⋯O) = 2.17(2)–2.37(2) Å]. By contrast, moderately weak, intermolecular N–H(amide)⋯O
C(amide) hydrogen bonds [d′(H⋯O) = 2.17(2)–2.37(2) Å]. By contrast, moderately weak, intermolecular N–H(amide)⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(ester) hydrogen bonds between the H2Et2mpba molecules are involved in 2 to give a meso-helical chain with a unique hydrogen-bonded oxalamide acid ester dimeric unit. The energy calculations as a function of the intermolecular N–H(amide)⋯O
C(ester) hydrogen bonds between the H2Et2mpba molecules are involved in 2 to give a meso-helical chain with a unique hydrogen-bonded oxalamide acid ester dimeric unit. The energy calculations as a function of the intermolecular N–H(amide)⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(ester) hydrogen bond distance (d′) for the {HMepma}2 dimer show an energy minimum at 2.37 Å, in excellent agreement with the experimental value of 2 [d′(H⋯O) = 2.42(4) Å]. The calculated value of the hydrogen bond energy for {HMepma}2 (EHB = 4.83 kcal mol−1) is consistent with a partially covalent nature of the interaction between the amide hydrogen and the carbonyl ester oxygen atoms, as confirmed by the existence of a significant electron density delocalization within the resulting four-center H2O2 diamond core.
C(ester) hydrogen bond distance (d′) for the {HMepma}2 dimer show an energy minimum at 2.37 Å, in excellent agreement with the experimental value of 2 [d′(H⋯O) = 2.42(4) Å]. The calculated value of the hydrogen bond energy for {HMepma}2 (EHB = 4.83 kcal mol−1) is consistent with a partially covalent nature of the interaction between the amide hydrogen and the carbonyl ester oxygen atoms, as confirmed by the existence of a significant electron density delocalization within the resulting four-center H2O2 diamond core.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(amide)
C(amide) ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(amide)
C(amide) ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(ester)
C(ester) ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(ester)
C(ester) 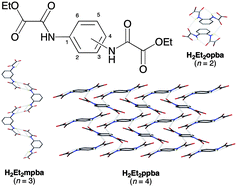

 Please wait while we load your content...
Please wait while we load your content...