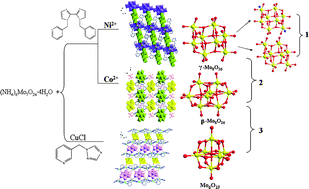
Three novel compounds co-existing isomers or forms of polymolybdate, namely, {[Ni(γ-Mo8O26)(H2L1)2(H2O)2](γ-Mo8O26)(H2L1)}·2H2O (1), {[Co(γ-Mo8O26)(H2L1)2(H2O)2](β-Mo8O26)[Co(H2O)6]}·6H2O (2) and [CuI6L24(β-Mo8O26)(Mo6O19)] (3), where L1 = 1,1′-bis(pyridin-3-ylmethyl)-2,2′-biimidazole, L2 = 3-((1H-1,2,4-triazol-1-yl)methyl)pyridine, have been successfully synthesized. Crystal structure analysis reveals that 1 is a 1D + 1D supramolecular structure containing both γ-[Mo8O26N2] and γ-[Mo8O28] units; 2 has γ-[Mo8O26]4− as well as β-[Mo8O26]4− anions and 3 is a (3,4,6)-connected 2D network containing both β-[Mo8O26]4− and [Mo6O19]2− anions. According to quantum chemical calculations, the transformation from β-[Mo8O26]4− to [Mo6O19]2− is spontaneous in aqueous solution. Furthermore, we have investigated the optical band gap of compounds 1 and 3, with 3 showing a lower band gap (1.69 eV). Additionally, the photoluminescent property of 3 is also studied, which can be assigned to metal-to-ligand charge transfer (MLCT).

 Please wait while we load your content...
Please wait while we load your content...