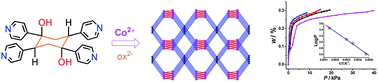
A novel three-dimensional (3D) nanoporous metal–organic framework with moganite topology, [Co2(ox)2(dchtpy)]n·9nH2O (1·9H2O), has been obtained from the hydrothermal reaction of CoCl2, oxalic acid, NaOH and 1a,4a-dihydroxy-1e,2e,4e,5e-tetra(4-pyridyl)cyclohexane (dchtpy). X-ray analysis reveals that the bulky dchtpy ligands link four adjacent metal oxalate chains and form a 3D hybrid network with both moganite topology and 3D channels in various sizes. The largest pores (channel A) are the ones viewed along the a-axis, which are composed of two [Co(ox)]n chains and two bulky ligands in the dimensions of 10.76 × 10.67 Å excluding the van der Waals radii. Along the {101} direction there was found the second large channels (channel B) in the size of 10.51 × 4.66 Å. Notably the hydroxyl groups of dchtpy extend towards the channels on the middle of the walls, dividing the pores with two small channels in approximately 4.50 × 4.66 Å. Besides, small pores in dimensions of 7.07 × 8.24 Å and 6.98 × 6.06 Å can also be found along the a- and c-axes, respectively. The potential solvent accessible void of the framework, calculated by PLATON, is 46.1% of the total volume and is occupied with disordered aqua molecules. Variable-temperature powder X-ray diffraction experiments confirmed that the crystallinity and network integrity of 1 can be retained up to 375 °C upon the removal of guest aqua molecules, in which thermal stability is among the most stable metal organic coordination frameworks. The framework shows guest-induced dynamic flexibility and elasticity and has a gating effect on the sorption of small gas molecules. The hydroxyl groups from the ligands functionalize the walls of the channels and have strong hydrogen bonds with adsorbents, leading to high sorption capacity at ambient temperature. Magnetic studies revealed significant antiferromagnetic interaction between the Co(II) ions in 1·9H2O with J = −12.4(1) cm−1 and g = 2.450(4).

 Please wait while we load your content...
Please wait while we load your content...