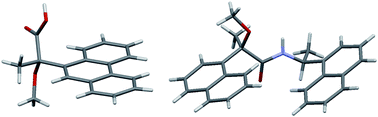
This paper reports the first evidence of the contribution of T-shaped aromatic CH⋯π interactions to the crystallization of 2-aryl-2-methoxypropanoic acid and amide. The crystal structures of (S)-2-methoxy-2-(9-phenanthryl)propanoic acid [(S)-M9PP acid, (S)-3] and a diastereomeric amide 6, prepared from (R)-2-methoxy-2-(1-naphthyl)propanoic acid [(R)-MαNP acid, (R)-1] and (S)-1-(1-naphthyl)ethylamine [(S)-4], were determined by X-ray crystallography. Like those of M9PP and MαNP esters, the carbonyl and methoxy groups of (S)-3 exhibited syn conformation. The crystal structure of (S)-3 was stabilized by consecutive intermolecular OH⋯O hydrogen bonds between the carboxy groups. In addition, the 9-phenanthryl groups formed a herringbone structure via aromatic CH⋯ π interactions. The anti conformation of the carbonyl and methoxy groups was observed in the crystal of amide 6, indicating an NH⋯O hydrogen bond. The two 1-naphthyl groups were parallel to one another and located on the same side of the MαNP plane, forming a herringbone structure with adjacent molecules. NMR analyses suggest that, in solution, intramolecular aromatic CH⋯π interactions induce the 1-naphthyl groups to form a slant-parallel conformation. Namely, both the length and interactions between the substituents are important for crystallization. Normal-phase HPLC separation of diastereomeric amides is also discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...