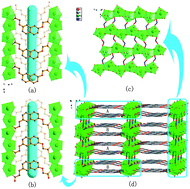
Eight new coordination complexes, namely [ZnL1(H2O)2]n (1), [CdL1(H2O)2]n (2), [CoL1(H2O)3]n (3), [EuL1(NO2)(H2O)]n (4), [SmL1(NO2)(H2O)]n (5), [CdL2(H2O)]n (6), [CoL2(H2O)4]n (7) and [NiL2(H2O)4]n (8) were synthesized under solvothermal conditions based on flexible ligands 2,2′-(naphthalene-2,7-diylbis(oxy)) diacetic acid (H2L1) and 2,2′-(biphenyl-4,4′-diylbis(oxy)) diacetic acid (H2L2). X-Ray diffraction analysis revealed that the eight complexes exhibited new frameworks due to diverse coordination conformations of the flexible bicarboxylate ligands. In complexes 1–8, H2L1 and H2L2 all acted as bridging ligands to connect the metal ions. Complexes 1 and 2 both exhibited three-demensional (3D) supramolecular structures formed by one-demensional (1D) chains through intermolecular interactions. Complex 3 formed its 3D network by connecting the two-demensional (2D) layers through hydrogen bonds. Complexes 4 and 5 had similar 3D frameworks which are combined by nitroso groups as pillars. Complex 6 represented a 3D framework with pcu α-Po topology by using L22− ligands as linkers and the [Cd2O6] secondary building units (SBUs) as 6-connected nodes. The isostructural complexes 7 and 8 also gave rise to 3D supramolecular networks by joining 1D chains with hydrogen bonds and π–π interactions. The luminescent properties for 1, 2, 4 and 6 are discussed in detail. Complexes 3, 4, 7 and 8 exhibited antiferromagnetic interactions between the magnetic centers.

 Please wait while we load your content...
Please wait while we load your content...