The crystal structure of racemic 2,6-di-O-benzoyl-myo-inositol 1,3,5-orthoformate (1) which underwent a facile intermolecular benzoyl transfer reaction in the solid state, revealed a helical assembly of molecules along the two-fold screw axis via O–H⋯O hydrogen bond bringing the electrophile (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and the nucleophile (–OH) in close proximity along the helical axis. However, structurally related racemic 2,6-di-O-(p-halobenzoyl)-myo-inositol 1,3,5-orthoformates (bromo (2) and chloro (3)) produced triclinic dimorphs (both P
O) and the nucleophile (–OH) in close proximity along the helical axis. However, structurally related racemic 2,6-di-O-(p-halobenzoyl)-myo-inositol 1,3,5-orthoformates (bromo (2) and chloro (3)) produced triclinic dimorphs (both P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) when crystallized from methanol and ethyl acetate. Molecules in either form did not assemble spirally (like 1), and instead exhibited a one-dimensional isostructurality, bridging O–H⋯O linked identical molecular strings via C–H⋯O interactions across the inversion center. However, the molecules of 2 and 3 assembled in a helical manner similar to 1 with inclusion of solvent molecules in the crystal lattice when crystallized from other common organic solvents. Remarkably, in all the solvates the host molecules formed strikingly similar helices around the crystallographic 21-screw axis through O–H⋯O bond involving the –OH group and carbonyl oxygen of the equatorial C2-O-benzoyl group. Comparison of the crystal structure of dimorphs and the solvatomorphs revealed that the solvent molecules, which interact with the orthoformate-bridge, trigger the helix formation of the host. The difference in the crystal structures of solvatomorphs arises in the interlinking of the neighbouring helices, which creates voids of different sizes to accommodate the solvent molecules. All the solvates crystallized in the monoclinic system distributed over three different space groups P21/n, P21/c and C2/c. In the P21/n system, the adjacent helices are linked via C–X⋯O contacts, in P21/c via C–H⋯X (X = Cl, Br) contacts and in C2/c via short X⋯X contacts (X = Cl). The helical organization achieved through solvent mediation and inclusion is of significance in creating molecular packing for intermolecular acyl transfer reactions in crystals.
) when crystallized from methanol and ethyl acetate. Molecules in either form did not assemble spirally (like 1), and instead exhibited a one-dimensional isostructurality, bridging O–H⋯O linked identical molecular strings via C–H⋯O interactions across the inversion center. However, the molecules of 2 and 3 assembled in a helical manner similar to 1 with inclusion of solvent molecules in the crystal lattice when crystallized from other common organic solvents. Remarkably, in all the solvates the host molecules formed strikingly similar helices around the crystallographic 21-screw axis through O–H⋯O bond involving the –OH group and carbonyl oxygen of the equatorial C2-O-benzoyl group. Comparison of the crystal structure of dimorphs and the solvatomorphs revealed that the solvent molecules, which interact with the orthoformate-bridge, trigger the helix formation of the host. The difference in the crystal structures of solvatomorphs arises in the interlinking of the neighbouring helices, which creates voids of different sizes to accommodate the solvent molecules. All the solvates crystallized in the monoclinic system distributed over three different space groups P21/n, P21/c and C2/c. In the P21/n system, the adjacent helices are linked via C–X⋯O contacts, in P21/c via C–H⋯X (X = Cl, Br) contacts and in C2/c via short X⋯X contacts (X = Cl). The helical organization achieved through solvent mediation and inclusion is of significance in creating molecular packing for intermolecular acyl transfer reactions in crystals.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and the nucleophile (–OH) in close proximity along the helical axis. However, structurally related racemic 2,6-di-O-(p-halobenzoyl)-myo-inositol 1,3,5-orthoformates (
O) and the nucleophile (–OH) in close proximity along the helical axis. However, structurally related racemic 2,6-di-O-(p-halobenzoyl)-myo-inositol 1,3,5-orthoformates (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) when crystallized from
) when crystallized from 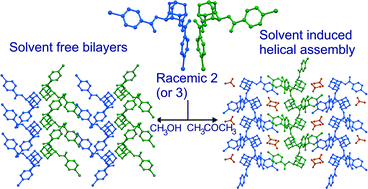

 Please wait while we load your content...
Please wait while we load your content...