Transannular, decarboxylative Claisen rearrangement reactions for the synthesis of sulfur-substituted vinylcyclopropanes†
Abstract
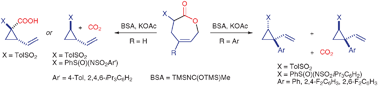
Unsaturated ε-lactones bearing an α-arylsulfonyl or α-arylsulfoximinyl substituent undergo stereoselective transannular, decarboxylative

 Please wait while we load your content...
Please wait while we load your content...