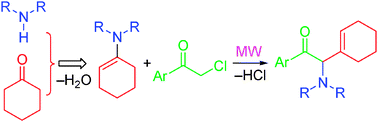
Solvent-free microwave-assisted multi-component reaction for preparation of 2-amino-1-aryl-2-(cyclohex-1-enyl)ethanones as precursors of pseudoephedrine analogues†
Abstract
Microwave irradiation of chloroacetylarenes and enamines induced an aza-Darzens reaction followed by

 Please wait while we load your content...
Please wait while we load your content...