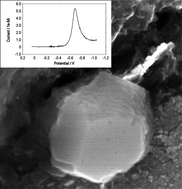
Electrochemical discrimination between dopamine and psychotropic drugs which have in common a skeletal structure of phenethylamine, can be obtained using acyclic receptors L1 and L2, containing two terminal 3-alkoxy-5-nitroindazole rings. Upon attachment to graphite electrodes, L1 and L2 exhibit a well-defined, essentially reversible solid state electrochemistry in contact with aqueous media, based on electrolyte-assisted reduction processes involving successive cation and anion insertion/binding. As a result, a distinctive, essentially Nernstian electrochemical response is obtained for phenethylammonium ions of methamphetamine (METH), p-methoxyamphetamine (PMA), amphetamine (AMPH), mescaline (MES), homoveratrylamine (HOM), phenethylamine (PEA) and dopamine (DA) in aqueous media.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...