Interaction of cationic surfactants with DNA detected by spectroscopic and acoustic wave techniques
Abstract
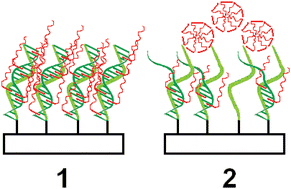
Interaction of the cationic
* Corresponding authors
a
Department of Nuclear Physics and Biophysics, Comenius University, Mlynska dolina F1, Bratislava, Slovakia
E-mail:
tibor.hianik@fmph.uniba.sk
Fax: +421-2-65426774
Tel: +421-2-60295683
b Department of Chemistry, University of Toronto, 80 St George Street, Toronto, Ontario, Canada
c Chemistry Department and A.N. Belozersky Institute of Physico-Chemical Biology, M.V. Lomonosov Moscow State University, Leninskije Gory, Moscow, Russia
d Institute of Experimental Physics, Slovak Academy of Sciences, Watsonova 47, Košice, Slovakia
e Tyndall National Institute, Lee Maltings, Prospect Row, Cork, Ireland
Interaction of the cationic

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
T. Hianik, X. Wang, V. Tashlitsky, T. Oretskaya, S. Ponikova, M. Antalík, J. S. Ellis and M. Thompson, Analyst, 2010, 135, 980 DOI: 10.1039/C0AN00070A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
