Cryophotolysis of a caged oxygen compound for use in low temperature biological studies†
Abstract
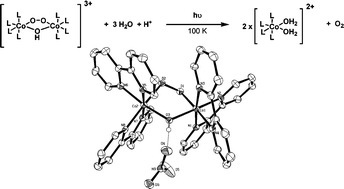
Mechanistic investigations of biological enzymatic processes require controlled initiation and monitoring of catalytic reactions. A well-known technique to trap and observe reaction intermediates building up along a reaction pathway is the use of low temperature conditions. Here, we report a kinetically competent system for the release of molecular oxygen at cryogenic temperature, using a cobalt-based caged oxygen molecule, (μ-peroxo)(μ-hydroxo)bis[bis(bipyridyl)cobalt(III)] nitrate. Cryophotolysis of this compound was induced using 266 nm laser light and monitored by absorption microspectrophotometry. Furthermore, to verify that photo-fragmentation was accompanied by release of the active caged molecule, the production of dioxygen during cryophotolysis was directly visualized. This work lays the foundations for the use of low temperature reaction triggering as a tool to prolong the lifetime of normally unstable intermediate states in oxygen-dependant enzymes.

 Please wait while we load your content...
Please wait while we load your content...