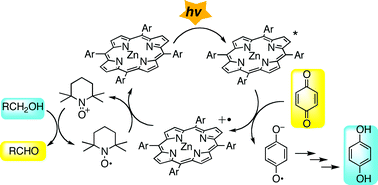
Photooxidation of alcohols to the corresponding aldehydes with a porphyrin/quinone/TEMPO (TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxy free radical) system is described. This photoreaction is a combination of a photoinduced electron transfer from the porphyrin to the quinone and a TEMPO-catalyzed oxidation of alcohols triggered by one electron oxidation. The rates of oxidation were in the order of benzylic ≈ allylic > primary ≫ secondary, which is consistent with the intermediacy of the oxoammonium cation derived from TEMPO. Examination of the initial rates suggested that the reaction proceeded via the triplet excited state of the zinc porphyrin. The dependence of initial rates on the oxidation potentials of the porphyrin showed a characteristic bell shape, which is caused by two competitive factors, the efficiency of photoinduced electron transfer and the equilibrium of electron exchange between the porphyrin cation radical and TEMPO. The potential significance of this reaction in photosynthetic model chemistry is briefly discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...