Enzymatic kinetic resolution of primary allenic alcohols. Application to the total synthesis and stereochemical assignment of striatisporolide A†
Abstract
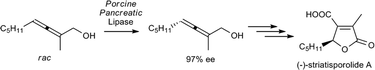
Crude Porcine pancreatic lipase was successfully used for the kinetic resolution of axially chiral primary allenic alcohols providing very high enantioselectivities with E values above 200. This simple access to optically active allenes was applied to the total synthesis of the fungal

 Please wait while we load your content...
Please wait while we load your content...