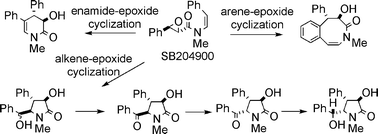
The synthesis of both antipodes of N-methyl-N-[(Z)-styryl]-3-phenyloxirane-2-carboxamide (SB204900), clausenamide, neoclausenamide, homoclausenamide and ζ-clausenamide have been accomplished using (2S,3R)- and (2R,3S)-3-phenyloxirane-2-carboxamides as the starting materials, and SB204900 was found to be a common precursor to other N-heterocyclic clausena alkaloids. Mediated by Brønsted acids under different conditions, for example, SB204900 underwent efficient and diverse alkene-epoxide cyclization, enamide-epoxide cyclization and arene-epoxide cyclization reactions to produce the five-membered N-heterocyclic neoclausenamide, its 6-epimer, the six-membered N-heterocyclic homoclausenamide and the eight-membered N-heterocyclic ζ-clausenamide, respectively, in good to excellent yields. Regiospecific oxidation of neoclausenamide and its 6-epimer afforded neoclausenamidone. Enolization of neoclausenamidone in the presence of LiOH and the subsequent protonation under kinetic conditions at −78 °C led to the epimerization of neoclausenamidone into clausenamidone. Reduction of clausenamidone using NaBH4 furnished clausenamide in high yield.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...