Direct metallation of thienopyrimidines using a mixed lithium–cadmium base and antitumor activity of functionalized derivatives†
Abstract
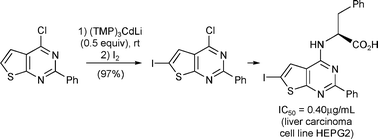
A series of
* Corresponding authors
a
Chimie et Photonique Moléculaires, UMR 6510 CNRS, Université de Rennes 1, Bâtiment 10A, Case 1003, Campus Scientifique de Beaulieu, Rennes, France
E-mail:
florence.mongin@univ-rennes1.fr
Fax: +33-2-2323-6955
b Laboratoire de Synthèse Organique Appliquée, Faculté des Sciences de l'Université, BP 1524 Es-Senia, Oran, Algeria
c Department of Chemistry, Faculty of Women for Arts, Science and Education, Ain Shams University, Asma Fahmy Street, Heleopolis (El-Margany), Cairo, Egypt
d Laboratoire d'Ingéniérie Moléculaire et Biochimie Pharmacologique, Institut Jean Barriol, FR CNRS 2843, Université Paul Verlaine-Metz, 1 Boulevard Arago, Metz Technopôle, France
A series of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
K. Snégaroff, F. Lassagne, G. Bentabed-Ababsa, E. Nassar, S. C. S. Ely, Stéphanie Hesse, E. Perspicace, A. Derdour and F. Mongin, Org. Biomol. Chem., 2009, 7, 4782 DOI: 10.1039/B915274A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
