4-Aminoproline-based arginine-glycine-aspartate integrin binders with exposed ligation points: practical in-solution synthesis, conjugation and binding affinity evaluation†
Abstract
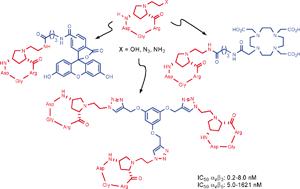
An expedient and practical in-solution synthesis of three new
* Corresponding authors
a
Dipartimento Farmaceutico, Università degli Studi di Parma, Viale G. P. Usberti 27A, I-43100 Parma, Italy
E-mail:
lucia.battistini@unipr.it, giovanni.casiraghi@unipr.it
Fax: +39-0521-905006
Tel: +39-0521-906040
b Istituto di Chimica Biomolecolare del CNR, Traversa La Crucca 3, I-07040 Li Punti, Sassari, Italy
c Centro Interdipartimentale Studi Biomolecolari e Applicazioni Industriali, Università degli Studi di Milano, Via Fantoli 16/15, I-20138 Milano, Italy
d Istituto di Scienze e Tecnologie Molecolari del CNR, Via Fantoli 16/15, I-20138 Milano, Italy
An expedient and practical in-solution synthesis of three new

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
L. Battistini, P. Burreddu, P. Carta, G. Rassu, L. Auzzas, C. Curti, F. Zanardi, L. Manzoni, E. M. V. Araldi, C. Scolastico and G. Casiraghi, Org. Biomol. Chem., 2009, 7, 4924 DOI: 10.1039/B914836A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
