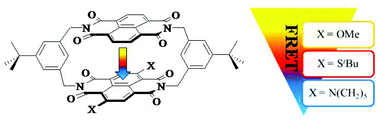
A series of planar chiral asymmetric naphthalenediimide (NDI) cyclophanes (1–4) consisting of two different NDIs interlinked by rigid meta-dimethylenebenzene spacers was synthesized. A modular synthetic strategy based on the dichloro cyclophane 1 as common precursor gave a straightforward access to racemic mixtures of the NDIcyclophane dyes 2–4. In particular the substitution of both chlorine atoms of 1 by tert-butylsulfanyl-, methoxy- and piperidinyl-groups provided racemic mixtures of the fluorescent NDI cyclophane dyes 2, 3 and 4 respectively. Fluorescence spectroscopy revealed chemically tuneable intramolecular FRET properties, as the spectral overlap between the emission of the unsubstituted NDI and the absorption of the core-substituted NDI subunit of the cyclophane could be adjusted by the core substituents. The racemic nature of the samples of cyclophanes 2–4, and in the case of the di-tert-butylsulfanyl functionalized cyclophane 2 the enrichment of both enantiomers, was demonstrated by HPLC using a chiral stationary phase. The solid state structure of 2 was determined by X-ray analysis. A periodic arrangement of both enantiomers of the planar chiral cyclophane 2 in pillars such that NDI- and 2,6-core substituted NDI chromophores alternate periodically was observed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...