On the origin of the regioselectivity in glycosylation reactions of 1,2-diols†
Abstract
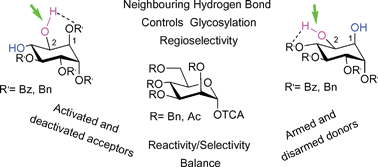
The assistance of neighboring protecting groups with different orientations in 1,2-diol acceptors and the reactivity of both reaction partners, the donor and the acceptor, have been evaluated as factors that determine the regioselectivity of glycosylation reactions. It has been established, by experimental and theoretical studies, that the regioselectivity for the glycosylation of a given OH group can be considerably increased by the presence of groups able to form a hydrogen bond with that OH group. Moreover higher regioselectivities are observed when armed donor/activated acceptor combinations are avoided.

 Please wait while we load your content...
Please wait while we load your content...