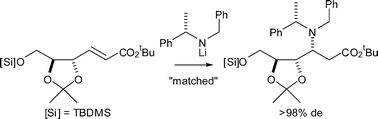
As part of a long-term goal directed towards the ab initio asymmetric synthesis of unnatural amino sugars, the doubly diastereoselective conjugate addition reactions of the antipodes of lithium N-benzyl-N-(α-methylbenzyl)amide to a range of homochiral α,β-unsaturated esters containing cis- and trans-dioxolane units was investigated. These reactions resulted in “matching” and “mismatching” effects. In the “matched” cases a single diastereoisomer of the corresponding β-amino ester (containing three contiguous stereocentres) is produced. Upon conjugate addition to a homochiral α,β-unsaturated ester containing a cis-dioxolane unit, in the “mismatched” case it is the stereocontrol of the substrate which is dominant over that of the lithium amide, whilst upon addition to homochiral α,β-unsaturated esters containing a trans-dioxolane unit the stereocontrol of the homochiral lithium amide is dominant. Hydrogenolytic N-deprotection of the β-amino ester products of conjugate addition gives access to polyoxygenated β-amino acid derivatives.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...