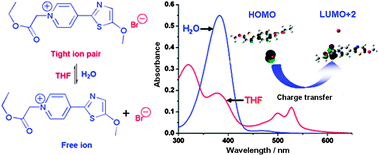
A new fluorophore, 1-(ethoxy-carbonyl-methyl)-4-[2-(5-methoxy)thiazolyl]pyridinium bromide (4-MeB), has been synthesized and its photophysical properties investigated. In solvents with a larger dielectric constant (ε), 4-MeB is ionized into free ions, showing a characteristic thiazolyl-pyridinium cation-centered π→π* electronic transition absorption band in the UV region. In solvents with smaller ε values, such as tetrahydrofuran (THF), 1,4-dioxane (dioxane) and ethyl acetate (EtOAc), 4-MeB exists in the form of a tight ion pair, and concomitantly displays characteristic bromide anion-dependent absorption in the visible and UV regions. Among several other anions, such as PF6−, I− and Cl−, and neutral electron donor Et3N, only I− shows a similar influence on the absorption spectrum of 4-Me++ to that of bromide anion, confirming the bromide-dependent spectral properties of 4-MeB. With the help of theoretical calculations, the absorption in visible region is attributed to the charge-transfer from bromide anion to thiazolyl-pyridinium cation, which is tuned by the distance between the bromide anion and the thiazolyl-pyridyl moiety. In all of the solvents employed, 4-MeB also exhibits a dual-emissive fluorescent property, and the position of its emission peak is influenced by the excitation wavelength.

 Please wait while we load your content...
Please wait while we load your content...