Fullerene-rich dendrimers: divergent synthesis and photophysical properties†
Abstract
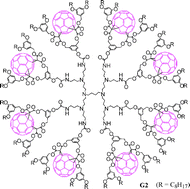
Dendrimers containing up to 16
- This article is part of the themed collection: In honour of Jean-Pierre Sauvage
* Corresponding authors
a
Laboratoire de Chimie des Matériaux Moléculaires, Ecole Européenne de Chimie, Polymères et Matériaux, Université Louis Pasteur et CNRS (UMR 7509), 25 rue Becquerel, Strasbourg Cedex 2, France
E-mail:
nierengarten@chimie.u-strasbg.fr
Fax: +33 390 242774
Tel: +33 390 242764
b
Kekulé-Institut für Organische Chemie und Biochemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, Bonn, Germany
E-mail:
voegtle@uni-bonn.de
Fax: +49 228 735662
Tel: +49 228 733496
c
Molecular Photoscience Group, Istituto per la Sintesi Organica e la Fotoreattività, Consiglio Nazionale delle Ricerche, via Gobetti 101, Bologna, Italy
E-mail:
nicola.armaroli@isof.cnr.it
Fax: +39 051 639 9844
Tel: +39 051 639 9820
Dendrimers containing up to 16

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
U. Hahn, J. Nierengarten, F. Vögtle, A. Listorti, F. Monti and N. Armaroli, New J. Chem., 2009, 33, 337 DOI: 10.1039/B816336G
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
