Reactions of alkali metal amides or phosphides with the bulky carbodiimide, ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr (Ar = C6H3Pri2-2,6), followed by aqueous work-ups, have yielded several guanidines, ArNC(NR2)N(H)Ar (R = cyclohexyl (GisoH) or Pri (PrisoH); NR2 = cis-NC5H8Me2-2,6 (PipisoH)), a bifunctional guanidine, {ArNCN(H)Ar}2{μ-N(C2H4)2N} (Pip(GisoH)2), and two phosphaguanidines, ArNC(PR2)N(H)Ar (R = cyclohexyl (CyP-GisoH) or Ph (PhP-GisoH)). A very bulky guanidine, ArNC{N(Ar)SiMe3}N(H)Ar (ArSi-Giso), and an aryl coupled bifunctional guanidine, {ArN(H)C(NPri2)NC6H2Pri2-2,6-}2 (PrisoH)2, have been prepared by other routes. All compounds have been crystallographically characterised and shown to exist in a number of isomeric forms in the solid state. These appear to be largely retained in solution. The deprotonation of GisoH with BunLi in either hexane or THF led to crystallographically characterised dimeric and monomeric complexes respectively, viz. [Li{Li(κ2-N,N′-Giso)2}] and [Li(THF)(η1-N,η3-Ar-Giso)]. Deprotonation of PrisoH and Pip(GisoH)2 with K[N(SiMe3)2] gave the unsolvated polymer, [{K(η1-N,η6-Ar-Priso)}∞], and the solvated complex, [{K(THF)2}{Pip(Giso)2}{K(THF)3}], respectively.
NAr (Ar = C6H3Pri2-2,6), followed by aqueous work-ups, have yielded several guanidines, ArNC(NR2)N(H)Ar (R = cyclohexyl (GisoH) or Pri (PrisoH); NR2 = cis-NC5H8Me2-2,6 (PipisoH)), a bifunctional guanidine, {ArNCN(H)Ar}2{μ-N(C2H4)2N} (Pip(GisoH)2), and two phosphaguanidines, ArNC(PR2)N(H)Ar (R = cyclohexyl (CyP-GisoH) or Ph (PhP-GisoH)). A very bulky guanidine, ArNC{N(Ar)SiMe3}N(H)Ar (ArSi-Giso), and an aryl coupled bifunctional guanidine, {ArN(H)C(NPri2)NC6H2Pri2-2,6-}2 (PrisoH)2, have been prepared by other routes. All compounds have been crystallographically characterised and shown to exist in a number of isomeric forms in the solid state. These appear to be largely retained in solution. The deprotonation of GisoH with BunLi in either hexane or THF led to crystallographically characterised dimeric and monomeric complexes respectively, viz. [Li{Li(κ2-N,N′-Giso)2}] and [Li(THF)(η1-N,η3-Ar-Giso)]. Deprotonation of PrisoH and Pip(GisoH)2 with K[N(SiMe3)2] gave the unsolvated polymer, [{K(η1-N,η6-Ar-Priso)}∞], and the solvated complex, [{K(THF)2}{Pip(Giso)2}{K(THF)3}], respectively.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr (Ar = C6H3Pri2-2,6), followed by aqueous work-ups, have yielded several
NAr (Ar = C6H3Pri2-2,6), followed by aqueous work-ups, have yielded several 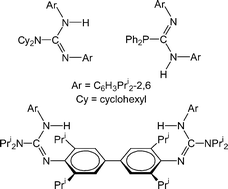

 Please wait while we load your content...
Please wait while we load your content...