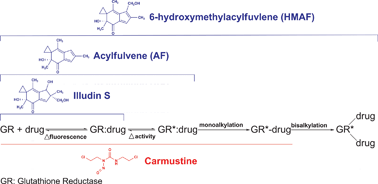
Acylfulvenes (AFs) are a class of antitumor agents with favorable cytotoxic selectivity profiles compared to their natural product precursor, illudin S. Like many alkylating agents, illudin S and AFs readily react with thiol-containing small molecules such as cysteine, glutathione and cysteine-containing peptides; reduced cellular glutathione levels can affect illudin S toxicity. Glutathione reductase (GR) is a critical cellular antioxidant enzyme that regulates the intracellular ratio of reduced–oxidized glutathione. In this study, we found that acylfulvene analogues are GRinhibitors, and evaluated aspects of the drug–enzyme interactions as compared with the structurally related natural productilludin S and the known irreversible GRinhibitor, carmustine. Acylfulvene analogues exhibited concentration-dependent GR inhibitory activity with micromolar IC50s; however, up to 2 mM illudin S did not inhibit GR activity. The absence of NADPH attenuates GR inhibition by AFs and the presence of glutathione disulfide (GSSG), the natural GR substrate, which binds to the enzyme active site, has a minimal effect in protecting GR from AFs. Furthermore, each compound can induce GR conformation changes independent of the presence of NADPH or GSSG. These results, together with gel-filtration analysis results and mass spectrometry data, indicate AF is a reversible inhibitor and HMAF an irreversible inhibitor that can form a bis-adduct with GR by reacting with active site cysteines. Finally in a cell-based assay, illudin S and HMAF were found to inhibit GR activity, but this inhibition was not associated with the reduction of GR levels in the cell. A model accounting for differences in mechanisms of GR inhibition by the series of compounds is discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...