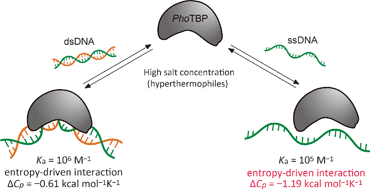
The interaction of hyperthermophilic TATA-box binding protein with single-stranded DNA is entropically favorable and exhibits a large negative heat capacity change at high salt concentration†
Abstract
We have investigated the thermodynamics of the interaction between the
- This article is part of the themed collection: Emerging investigators

 Please wait while we load your content...
Please wait while we load your content...