Integrated microfluidic chip for endothelial cells culture and analysis exposed to a pulsatile and oscillatory shear stress†
Abstract
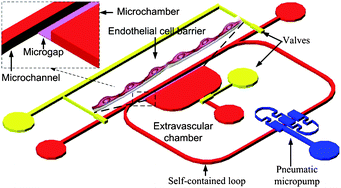
For a comprehensive understanding of cells or tissues, it is important to enable multiple studies under the controllable microenvironment of a chip. In this report, we present an integrated microfluidic cell culture platform in which endothelial cells (ECs) are under static conditions or exposed to a pulsatile and oscillatory shear stress. Through the integration of a microgap, self-contained flow loop, pneumatic pumps, and valves, the novel microfluidic chip achieved multiple functions: pulsatile and oscillatory fluid circulation, cell trapping, cell culture, the formation of ECs barrier, and adding shear stress on cells. After being introduced into the chip by gravity, the ECs arranged along the microgap with the help of hydrodynamic forces and grew in the microchannel for more than 7 days. The cells proliferated and migrated to form a barrier at the microgap to mimic the vessel wall, which separated the microenvironment into two compartments, microchannel and microchamber. An optimized pneumatic micropump was embedded to actuate flow circulation in a self-contained loop that induced a pulsatile and oscillatory shear stress at physiological levels on the ECs in the microchannel. All the analyses were performed under either static or dynamic conditions. The performance of the barrier was evaluated by the diffusion and distribution behaviors of fluorescently labeled albumin. The permeability of the barrier was comparable to that in traditional in vitro assays. The concentration gradients of the

 Please wait while we load your content...
Please wait while we load your content...