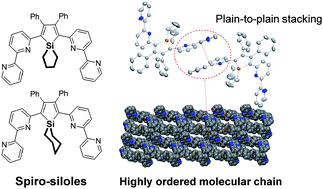
Two 2,5-bipyridyl substituted 1,1′-silanylene unit cyclized siloles with 1,1-silacyclopentyl- or 1,1-silacyclohexyl groups (3-Cy5 and 3-Cy6) were prepared from the intramolecular reductive cyclization of silacycloalkyl bis(phenylethynyl)silane with lithium naphthalenide and a a subsequent Pd-catalyzed cross-coupling reaction. Non-cyclized bipyridyl substituted dimethylsilole, 2,5-bis(2′,2′′-dipyridin-6-yl)-1,1-dimethyl-3,4-diphenylsilacyclopentadiene (PyPySPyPy), was also synthesized for comparison. All three siloles were characterized by X-ray structural studies. The results showed that the major contribution of three dimensional ordering found in crystal packing originated from distinctive intermolecular C–H⋯π interactions within the distance range 3.30–3.65 Å. Even higher ordering was apparent in the crystal packing due to the additional plane-to-plane packing interactions between the peripheral bipyridyl units in cyclized siloles, 3-Cy5 and 3-Cy6. In accordance with such an increase in the peripheral intermolecular interactions, 3-Cy5 and 3-Cy6 exhibited higher Tg values (95 and 86 °C, respectively) than the acyclic analogue, PyPySPyPy (77 °C). In particular, 3-Cy5 showed a higher electron mobility of 6.9 × 10−4 cm2V−1 s−1 at E = 0.581 MV cm−1 in the solid films. As a result, enhanced OLED performance was observed when 3-Cy5 was used as an electron transporting layer in the multilayered device structure of ITO/PEDOT·PSS/NPB/Alq3/3-Cy5/LiF/Al, showing a maximum luminance of 17430 cd m−2 at 11.5 V and a current efficiency of 4.52 cd A−1 at 69.4 mA cm−2 with a turn-on voltage of 2.6 V.

 Please wait while we load your content...
Please wait while we load your content...