Well-ordered periodic mesoporous organosilicas (PMOs) of the MCM-41 type of structure containing framework Ni(II) complexes (Ni@PMOs) were synthesized via a one-pot synthesis route in mild conditions, using cheap and environmental friendly reactants. These materials were obtained using the N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (AAPTMS) ligand in three different molar ratios of Ni, X = 1, 2, 3 and co-condensed with sodium silicate in the presence of cetyltrimethylammonium tosylate as a templating agent. The latter named OP-Ni-1, OP-Ni-2 and OP-Ni-3, respectively, were submitted to a treatment using a mixture of chlorotrimethylsilane (CTMS) and hexamethyldisilazane (HMDSA) that simultaneously removed the surfactant and capped the silanol groups leading to materials OP-Ni-1TA, OP-Ni-2TA and OP-Ni-3TA (∼2 wt% Ni content). For comparison, a series of mesoporous materials were prepared via a post-synthesis route to locate the Ni(II) complexes in the channel (∼2.4 wt% Ni). The latter were prepared using the “molecular stencil patterning” (MSP) technique based on sequential grafting of trimethylsilyl (TMS) groups and the same ligand cited above using AAPTMS/Ni molar ratios of 2 and 3. These steps lead to intermediate materials LUS to LUS-PSE and final materials LUS-MSP-Ni-2 and LUS-MSP-Ni-3. Both series of materials were thoroughly investigated in terms of (i) coordination state compared to molecular analogues, (ii) Ni(II) to Cu(II) exchange ability and (iii) coordination accessibility using ethylenediamine (en) or thiocyanate (SCN−) as probe ligands. All materials were investigated using XRD, TEM, N2 sorption isotherms, UV-visible, FT-IR and EPR spectroscopies. In particular, FT-IR and EPR data were treated quantitatively to monitor both the TMS loading and the concentration of isolated Cu(II) in conjunction with elemental analysis. Pore size decrease, pore volume reduction, nickel to copper exchangeability (> 90%) and ligand accessibility were observed for materials LUS-MSP-Ni-2 and LUS-MSP-Ni-3 as expected with grafted Ni(II) complexes located in the channel. By contrast, a clear opposite trend, observed for Ni@PMOs, OP-Ni-1, OP-Ni-2 and OP-Ni-3, was fully consistent with a complete insertion and captation of the Ni complexes in the siliceous pore walls despite the very small thickness (ca. 1.4 nm) of the wall. This is explained by the state of Ni(II) both in terms of coordination and cavity effect. Indeed, both absence of counterion coming from the solution (nitrate) and bathochromic shift of the d-d electronic transitions, are consistent with the presence of silanolate groups counterbalancing the electrical charges and mainly leading to neutral framework species of formula, [Ni(AAPS)2(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO)2], [Ni(AAPS)(OL)2(
SiO)2], [Ni(AAPS)(OL)2(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO)2], with neutral ligand OL = H2O,
SiO)2], with neutral ligand OL = H2O, ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiOH,
SiOH, ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiOSi
SiOSi![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) and AAPS = N-(2-aminoethyl)-3-aminopropylsilyl moities.
and AAPS = N-(2-aminoethyl)-3-aminopropylsilyl moities.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO)2], [Ni(AAPS)(OL)2(
SiO)2], [Ni(AAPS)(OL)2(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO)2], with neutral
SiO)2], with neutral ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiOH,
SiOH, ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiOSi
SiOSi![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) and AAPS = N-(2-aminoethyl)-3-aminopropylsilyl moities.
and AAPS = N-(2-aminoethyl)-3-aminopropylsilyl moities.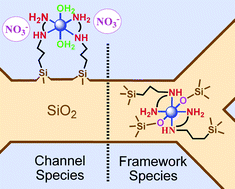

 Please wait while we load your content...
Please wait while we load your content...