Combined experimental and computational modelling studies of the solubility of nickel in strontium titanate†
Abstract
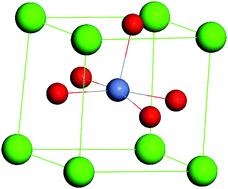
A combination of X-ray techniques and atomistic computational modelling has been used to study the solubility of Ni in SrTiO3 in relation to the application of this material for the catalytic partial
- This article is part of the themed collection: Dedicated to Professor C. N. R. Rao

 Please wait while we load your content...
Please wait while we load your content...