Reflection colour changes in cholesteric liquid crystals after the addition and photochemical isomerization of mesogenic azobenzenes tethered to sugar alcohols†
Abstract
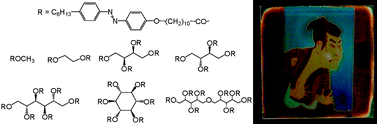
We controlled reflection colour of the glass-forming cholesteric liquid crystals over the whole visible region using the smectic liquid crystalline photochromic dopants on the basis of the different mechanism from the conventional helical twisting power change, which gave rise to full-colour recording and display materials at a lower additive concentration than that reported previously. We simply tethered multiple mesogenic azobenzene units to sugar alcohols to obtain effective dopants in which the intramolecular mesogenic moieties could adopt smectic-like alignments. The new dopants that featured linear sugar alcohol backbones exhibited increasingly stable smectic phases upon increasing the number of mesogenic groups in the molecule; a corresponding cyclic sugar alcohol derivative exhibited no such smectic phase. Addition of these smectic liquid crystalline dopants to the glass-forming cholesteric liquid crystalline material induced increasingly larger pitch shifts upon increasing the number of intramolecular mesogenic groups in the dopant. Dopants prepared from stereoisomeric sugar alcohol backbones provided similar liquid crystalline phases and induced similar pitch shifts after their addition to the cholesteric liquid crystals. The colour images recorded on the thin layer of the cholesteric liquid crystalline mixture were stabilized by fixing the cholesteric alignment into glassy states, which withstood heating at 70 °C.

 Please wait while we load your content...
Please wait while we load your content...