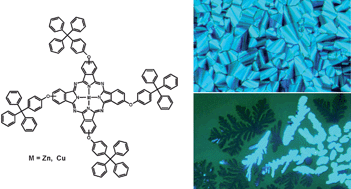
We describe the synthesis of highly soluble, tetrasubstituted zinc and copper phthalocyanines exhibiting liquid crystalline properties. These compounds were characterized using elemental analysis, MS, 1H-NMR, FTIR and UV-Vis absorption spectroscopy. The liquid crystalline properties of phthalocyanines (Pcs) were studied by polarizing optical microscopy and differential scanning calorimetry. All of the four compounds exhibit thermotropic columnar mesophases and two of them show clear hexagonal ordering. Additionally, these Pcs display lyotropic behaviour. The clearing temperatures lie above 300 °C and on cooling down the mesophase can be “frozen” into a glassy state. The cyclic voltammetry studies of these phthalocyanines show electrochemical stability and reversible redox behaviour. The Pcs without any azo groups in the ligands exhibit both reversible oxidation and reduction, whereas those with azo groups show only reversible reduction steps. The low HOMO energy values of about 5 eV as calculated from oxidation potential make these compounds interesting as p-type organic semiconductors for electro-optical applications.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...