Asymmetric hydrogenation using Wilkinson-type rhodium complexes immobilized onto mesoporous silica†
Abstract
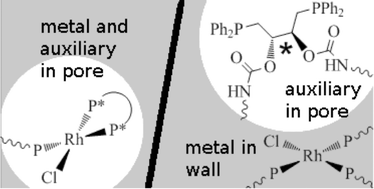
Heterogeneous chiral
* Corresponding authors
a
Laboratoire de Chimie, UMR 5182 ENS/CNRS, Ecole Normale Supérieure de Lyon, 46 allée d'Italie, F-69364 Lyon Cedex 07, France
E-mail:
vdufaud@ens-lyon.fr
Fax: +33 4727 28860
Tel: +33 4727 28857
b
ICBMS, UMR-CNRS 5246, Equipe Synthèse Asymétrique, Université de Lyon, Université Lyon 1, 43 boulevard du 11 Novembre, Villeurbanne cedex, France
E-mail:
framery@univ-lyon1.fr
Fax: +33 4724 48160
Tel: +33 4724 46263
Heterogeneous chiral

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
R. Sayah, E. Framery and V. Dufaud, Green Chem., 2009, 11, 1694 DOI: 10.1039/B915563P
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
