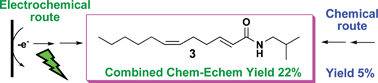
In order to show the advantages and limitations of organic electrosynthesis in the total synthesis of a natural product, one of the promising green chemistry techniques in organic chemistry, the synthesis of N-isobutyl-(2E,6Z)-dodecadienamide (3) was undertaken. Chemical and electrochemical routes that use the same intermediates were used to carry out the syntheses. Four reactions were compared from a green chemistry point of view in the synthesis of 3: (a) alcohol to aldehyde oxidation, (b) the Horner–Emmons reaction, (c) carboxylic acid amidation with triphenylphosphonium ions and (d) the Wittig reaction. All the electrolyses were carried out in non-divided cells at a constant current. The electrochemical method in the oxidation reaction of alcohols and the carboxylic acid amidation gave better yields (95% and 67%, respectively) than the corresponding chemical reactions. The Horner–Emmons reaction gave the same yields in both techniques (80–85%); however, the electrochemical method was more environmentally friendly, due to the fact that the base used was electrogenerated, avoiding corrosive and sensitive base manipulation. Finally, the electrochemical Wittig reaction was unsuccessful in the different experimental conditions attempted, and only the chemical method produced the target product. This study demonstrated that organic electrochemistry can be a reliable method for the synthesis of important intermediates, but not all electrochemical reactions can compete with the already well-established methods of organic chemistry.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...