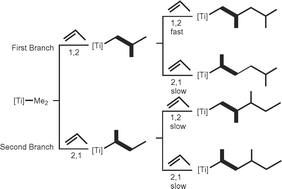
Some time ago we reported the quasi-living polymerization of propene with the catalytic mixture of Cp*TiMe3 and B(C6F5)3 (Cp* = C5Me5). Surprisingly, this mixture is extremely sensitive towards the nature of the anion and the presence of aluminium alkyl. This intriguing observation led us to the attempt to unearth the underlying reaction mechanism using a computational approach. In this communication, we are reporting the first results with the ‘naked cation’ approach. We obtained evidence, that the 1,2 insertion is the predominant reaction pathway. Whereas initial 1,2 and 2,1 insertion barriers are comparable, consequent second insertion is more discriminating between the two. Although we obtained evidence for the formation of β-H agostic bonds, we found that β-H elimination is a rare event due to the rather high activation barrier. We can conclude that the quasi-living polymerisation is at least partly an intrinsic property of the cation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...