Atropochiral (C,C)-chelating NHC-ylide ligands: synthesis and resolution of palladium(ii) complexes thereof†
Abstract
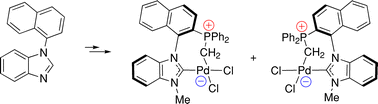
Atropochiral NHC-phosphonium ylides based on the
- This article is part of the themed collection: N-heterocyclic carbenes

 Please wait while we load your content...
Please wait while we load your content...