Synthesis and structure of early transition metal NHC complexes†
Abstract
The synthesis and structure of several new early transition metal (Ti, Zr, Hf, and V) NHC complexes supported by one or two IMes ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-2,6-iPr2-C6H3)Cl2(NHMe2)(IMes) (M = V (9), Zr (10)) are prepared from V(
N-2,6-iPr2-C6H3)Cl2(NHMe2)(IMes) (M = V (9), Zr (10)) are prepared from V(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR)Cl2(NHMe2)2 or ZrCl2(NMe2)2(IMes) synthons. Ionic imido complexes [M(
NR)Cl2(NHMe2)2 or ZrCl2(NMe2)2(IMes) synthons. Ionic imido complexes [M(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-2,6-iPr2-C6H3)Cl3(IMes)]−[HIMes]+ (M = V (12), Ti (13)) are obtained in modest yields from the treatment of [M(
N-2,6-iPr2-C6H3)Cl3(IMes)]−[HIMes]+ (M = V (12), Ti (13)) are obtained in modest yields from the treatment of [M(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR)Cl2(NHMe2)2] with 2 equiv. of IMes. Treatment of a
NR)Cl2(NHMe2)2] with 2 equiv. of IMes. Treatment of a ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)Cl2(NHMe2)2 (13), which reacts with two equiv. of IMes to give the bis-NHC
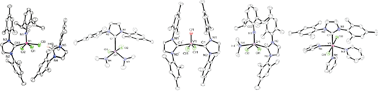
O)Cl2(NHMe2)2 (13), which reacts with two equiv. of IMes to give the bis-NHC ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)Cl2(IMes)2 (14). All new compounds have been characterised by various spectroscopic techniques, elemental analyses, and magnetism studies for paramagnetic compounds. The solid-state structure of 12 of these complexes and of IMes·HCl have further been confirmed by
O)Cl2(IMes)2 (14). All new compounds have been characterised by various spectroscopic techniques, elemental analyses, and magnetism studies for paramagnetic compounds. The solid-state structure of 12 of these complexes and of IMes·HCl have further been confirmed by
- This article is part of the themed collection: N-heterocyclic carbenes

 Please wait while we load your content...
Please wait while we load your content...