Chiral diphosphinites derived from 2,2-biphosphole as a new class of stereodynamic ligands for enantioselective hydrogenation†
Abstract
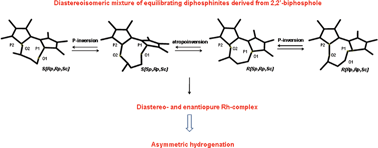
New stereodynamic diphosphinites derived from 2,2′-biphosphole, were synthesised by introduction of a linker obtained from chiral

 Please wait while we load your content...
Please wait while we load your content...