New 2-arylalkynyl benzo-1,3,2-diazaboroles, 2-(4′-XC6H4C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)-1,3-Et2-1,3,2-N2BC6H4 (X =Me 2; MeO 3; MeS 4; Me2N 5), were prepared from B-bromodiazaborole, 2-Br-1,3-Et2-1,3,2-N2BC6H4, with the appropriate lithiated arylacetylene, ArC
C)-1,3-Et2-1,3,2-N2BC6H4 (X =Me 2; MeO 3; MeS 4; Me2N 5), were prepared from B-bromodiazaborole, 2-Br-1,3-Et2-1,3,2-N2BC6H4, with the appropriate lithiated arylacetylene, ArC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CLi. Molecular structures of 2, 3 and 5 were determined by X-ray diffraction studies. UV-vis and luminescence spectroscopic studies on these diazaboroles reveal intense blue/violet fluorescence with very large quantum yields of 0.89–0.99 for 2–5. The experimental findings were complemented by DFT and TD-DFT calculations. The Stokes shift of only 2600 cm−1 for 5, compared to Stokes shifts in the range of 5900–7300 cm−1 for 1–4, is partly explained by the different electronic structures found in 5 compared to 1–4 (X = H). The HOMO is mainly located on the aryl group in 5 and on the diazaborolyl group in 1–4 whereas the LUMOs are largely aryl in character for all compounds. Thus, in contrast to other conjugated systems containing three-coordinate boron centers such as B(Mes)2, (Mes = 2,4,6-Me3C6H2), in which the boron serves as a π-acceptor, the 10-π electron benzodiazaborole moiety appears to function as a π-donor moiety.
CLi. Molecular structures of 2, 3 and 5 were determined by X-ray diffraction studies. UV-vis and luminescence spectroscopic studies on these diazaboroles reveal intense blue/violet fluorescence with very large quantum yields of 0.89–0.99 for 2–5. The experimental findings were complemented by DFT and TD-DFT calculations. The Stokes shift of only 2600 cm−1 for 5, compared to Stokes shifts in the range of 5900–7300 cm−1 for 1–4, is partly explained by the different electronic structures found in 5 compared to 1–4 (X = H). The HOMO is mainly located on the aryl group in 5 and on the diazaborolyl group in 1–4 whereas the LUMOs are largely aryl in character for all compounds. Thus, in contrast to other conjugated systems containing three-coordinate boron centers such as B(Mes)2, (Mes = 2,4,6-Me3C6H2), in which the boron serves as a π-acceptor, the 10-π electron benzodiazaborole moiety appears to function as a π-donor moiety.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)-1,3-Et2-1,3,2-N2BC6H4 (X =Me 2; MeO 3; MeS 4; Me2N 5), were prepared from
C)-1,3-Et2-1,3,2-N2BC6H4 (X =Me 2; MeO 3; MeS 4; Me2N 5), were prepared from ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CLi. Molecular structures of 2, 3 and 5 were determined by
CLi. Molecular structures of 2, 3 and 5 were determined by 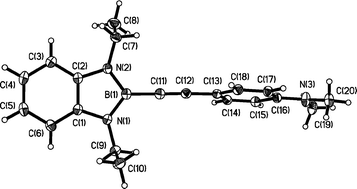

 Please wait while we load your content...
Please wait while we load your content...