Synthesis of a 1,4-benzodiazepine containing palladacycle with in vitro anticancer and cathepsin B activity†
Abstract
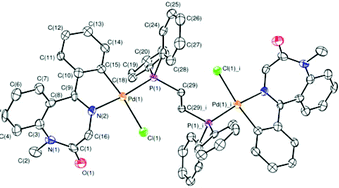
The reaction of the five-membered C,N-palladacycle [(L)PdCl]2, where LH = 1-methyl-5-phenyl-1H-1,4-benzodiazepin-2(3H)-one, with 1,2-ethanebis(diphenylphosphine),
- This article is part of the themed collection: Bioorganometallic chemistry

 Please wait while we load your content...
Please wait while we load your content...