Noble metal ionic catalysts: correlation of increase in CO oxidation activity with increasing effective charge on Pd ion in Pd ion substituted Ce1−xMxO2−δ (M = Ti, Zr and Hf)
Abstract
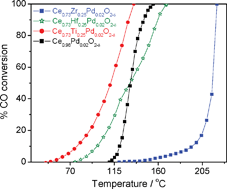
Pd ion substituted Ce1−xMxO2−δ (M = Ti, Zr, Hf) have been prepared by a single step solution

 Please wait while we load your content...
Please wait while we load your content...