The compounds OsCl2L2 (1), RuCl2L2 (2) and Ru(acac)2L (3), L° = N-phenyl-o-quinonediimine and acac− = 2,4-pentanedionato, have been synthesised and electrochemically, spectroscopically and structurally characterised with the support of detailed DFT calculations. For the new osmium compound 1, the average values for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH (1.331 Å), C
NH (1.331 Å), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh (1.345 Å) and C
NPh (1.345 Å) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(meta) bond lengths at 1.360 Å agree more with an o-semiquinonediimine than with an o-quinonediimine formulation for the coordinated ligand, whereas the latter assignment is more appropriate to describe the ruthenium analogue 2 (average bond lengths of 1.308, 1.324 and 1.348 Å, respectively). In turn, the corresponding average bond lengths in 3 (1.333, 1.352 and 1.350 Å, respectively) are suggestive of an o-semiquinonediimine ligand. DFT calculations confirm the structural data and describe the electronic structures related to the oxidation state assignments. The stabilisation of trivalent ruthenium by electron donating acac− in 3 = RuIII(acac)2(L•−) and the preference of osmium for higher oxidation states (here: OsIVCl2(L•−)2 in 1) as compared to ruthenium analogues (here: RuIICl2(L°)2 in 2) in otherwise similar setting is responsible for these results. Oxidation of the diamagnetic complexes proceeds to partly EPR and UV-VIS spectroelectrochemically detectable cations with metal-centered spin, suggesting the following formulations: [OsIIICl2(L°)2]+ for 1+, [RuIIICl2(L°)2]+ for labile 2+, and [RuIII(acac)2(L°)]+ for 3+. In the case of 3, reversible metal-centered reduction results in ligand-based spin as compatible with a [RuII(acac)2(L•−)]− situation for 3−.
C(meta) bond lengths at 1.360 Å agree more with an o-semiquinonediimine than with an o-quinonediimine formulation for the coordinated ligand, whereas the latter assignment is more appropriate to describe the ruthenium analogue 2 (average bond lengths of 1.308, 1.324 and 1.348 Å, respectively). In turn, the corresponding average bond lengths in 3 (1.333, 1.352 and 1.350 Å, respectively) are suggestive of an o-semiquinonediimine ligand. DFT calculations confirm the structural data and describe the electronic structures related to the oxidation state assignments. The stabilisation of trivalent ruthenium by electron donating acac− in 3 = RuIII(acac)2(L•−) and the preference of osmium for higher oxidation states (here: OsIVCl2(L•−)2 in 1) as compared to ruthenium analogues (here: RuIICl2(L°)2 in 2) in otherwise similar setting is responsible for these results. Oxidation of the diamagnetic complexes proceeds to partly EPR and UV-VIS spectroelectrochemically detectable cations with metal-centered spin, suggesting the following formulations: [OsIIICl2(L°)2]+ for 1+, [RuIIICl2(L°)2]+ for labile 2+, and [RuIII(acac)2(L°)]+ for 3+. In the case of 3, reversible metal-centered reduction results in ligand-based spin as compatible with a [RuII(acac)2(L•−)]− situation for 3−.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH (1.331 Å), C
NH (1.331 Å), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh (1.345 Å) and C
NPh (1.345 Å) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(meta) bond lengths at 1.360 Å agree more with an
C(meta) bond lengths at 1.360 Å agree more with an 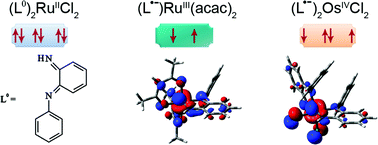

 Please wait while we load your content...
Please wait while we load your content...