Unique two-fold interpenetration of 3D microporous 3d–4f heterometal–organic frameworks (HMOF) based on a rigid ligand†
Abstract
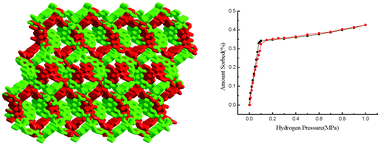
Six heterometal–organic frameworks, {[LnCu1.5(BPDC)3(H2O)2]·mH2O}n (Ln = Gd (1, m = 4.75), Tb (2, m = 3.75), Eu (3, m = 5), Ho (4, m = 2), Yb (5, m = 2.25), BPDC = 4,4′-dicarboxylate-2,2′-dipyridine anion), and {[LuCu1.5(BPDC)3(H2O)1.5]·2.5H2O}n (6), have been synthesized under hydrothermal conditions. The structure analyses for 1–6 reveal that the six compounds are isomorphous, belonging to the monoclinic system with space groupC2/c, and display two-fold interpenetrating 3D frameworks, which are the first examples of d–f heterometal–organic frameworks based on a rigid

 Please wait while we load your content...
Please wait while we load your content...