The electronic states of CeCp3+ have been studied experimentally by variable photon energy photoelectron spectroscopy, and computationally using multi-configurational ab initio methods. Relative partial photoionisation cross section and branching ratio data are presented to confirm our previous conclusion that bands A and D in the valence photoelectron spectrum, despite their 3.2 eV separation, are produced by ionization of the single 4f electron of CeCp3 [M. Coreno, M. de Simone, J. C. Green, N. Kaltsoyannis, N. Narband and A. Sella, Chem. Phys. Lett., 432, 2006, 17]. The origin of this effect is probed using the CASSCF/CASPT2 approach. While configurations based on the canonical CASSCF orbitals are found to be an unreliable description of the ground and excited states of CeCp3+, the state-specific natural orbitals and their occupations yield greater insight, allowing us to characterize ion states in terms of the presence or otherwise of a Ce 4f-localised electron. Neither the CeCp3+ ground state (assigned to band A), and two excited states (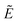 1A′ and 1A″, associated with band D), possess such a metal-based electron, as expected of f ionization. The
1A′ and 1A″, associated with band D), possess such a metal-based electron, as expected of f ionization. The 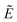 1A′ and 1A′′ states differ from the ground state in having a significant Ce 5d population, arising from Cp → Ce charge transfer, which accompanies f ionization, and which is responsible for the energetic separation of bands A and D in the valence photoelectron spectrum.
1A′ and 1A′′ states differ from the ground state in having a significant Ce 5d population, arising from Cp → Ce charge transfer, which accompanies f ionization, and which is responsible for the energetic separation of bands A and D in the valence photoelectron spectrum.
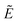 1A′ and 1A″, associated with band D), possess such a metal-based electron, as expected of f ionization. The
1A′ and 1A″, associated with band D), possess such a metal-based electron, as expected of f ionization. The 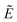 1A′ and 1A′′ states differ from the ground state in having a significant Ce 5d population, arising from Cp → Ce charge transfer, which accompanies f ionization, and which is responsible for the energetic separation of bands A and D in the valence photoelectron spectrum.
1A′ and 1A′′ states differ from the ground state in having a significant Ce 5d population, arising from Cp → Ce charge transfer, which accompanies f ionization, and which is responsible for the energetic separation of bands A and D in the valence photoelectron spectrum.

 Please wait while we load your content...
Please wait while we load your content...