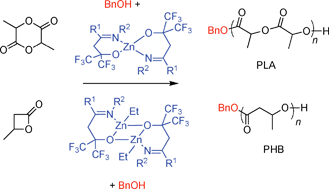
The reaction of fluorous imino-alcohols {ONR1,R2}H (R1 = Me, Ph; R2 = Ph, CH2Ph, cyclohexyl, piperidinyl) with Zn(N(SiMe3)2)2 (1 or 0.5 equiv) systematically led to bis(ligand) complexes Zn{ONR1,R2}2 (1–3), which were isolated in 84–88% yield. Imino-alcohols {ONR1,R2}H react selectively with ZnEt2 to give the corresponding complexes {ONR1,R2}ZnEt (4–7), in 74–78% yield. The stability of complexes 4–7 appears to be controlled by the nature of the substituent at the imine N atom: a phenyl group leads to slow ligand redistribution while alkyl-type substituents (benzyl, cyclohexyl, piperidinyl) prevent this disproportionation. Treatment of 5 with isopropanol afforded {ONPh,Bn}Zn(OiPr) (8) in modest yield, due to rapid ligand redistribution. Complexes 1–8 were authenticated by elemental analysis, 1H, 19F and 13C NMR spectroscopy in solution, and single-crystal X-ray diffraction studies for 1, 3, 5, 7 and 8. In situ combinations of {ONPh,Bn}ZnEt (5)/BnOH and Zn{ONPh,Bn}2 (2)/BnOH are active systems for the ROP of racemic lactide and β-butyrolactone at 20–50 °C, yielding atactic polylactide and poly(3-hydroxybutyrate) with good molecular weight control (Mn up to 20 700 g/mol) and relatively narrow molecular weight distributions (Mw/Mn = 1.06–1.57). These binary systems also allow the immortal ROP of lactide and β-butyrolactone, using excess alcohol (up to 5 equiv) vs the Zn catalyst.

 Please wait while we load your content...
Please wait while we load your content...