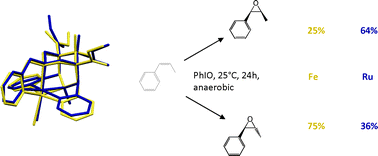
The synthesis and the full characterization of a new ruthenium(II) complex with the pentadentate bispidine ligand L1 is reported and shown to be a very active catalyst for olefin epoxidation. The selectivity in the epoxidation of cis- and trans-β-methylstyrene with the formation of cis and trans products, each, was determined and compared with that of the iron bispidine complex of L1. There is a significant difference in selectivity between the two catalysts in the epoxidation of cis-β-methylstyrene but the epoxidation of trans-β-methylstyrene is highly stereoselective with both catalysts. Based on these results, electrochemical and labeling studies, a radical pathway for the epoxidation and isomerization is proposed, and this is supported by computational data. DFT indicates that, with both catalysts, the epoxidation is based on a stepwise mechanism, which, in the first step leads to a radical-based intermediate. This exists in two configurations, which interconvert with a relatively low energy barrier. The product ratio depends on the relative energies of the two configurations of the radical intermediate and the height of the energy barriers to the cis- and trans-epoxide products. For the Fe-based system there is, as expected, the additional complication of the availability of various spin levels, and multi-state reactivity is observed. The computed structures and energies are in agreement with the observed data.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...