Spin canting and/or metamagnetic behaviours of four isostructural grid-type coordination networks†
Abstract
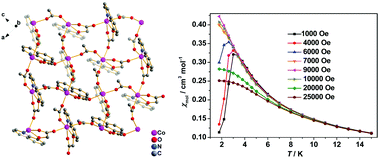
Four isostructural, two-dimensional (2D) grid-type coordination polymers bridged by carboxylate groups, namely [Co(8-qoac)(HCOO)] (1·Co), [Mn(8-qoac)(HCOO)] (1·Mn), [Co(8-qoac)(CH3COO)] (2·Co) and [Mn(8-qoac)(CH3COO)] (2·Mn) (8-qoacH = quinoline-8-oxy-acetate acid), were constructed to study the modulation effects of spin carriers and interlayer interactions on the magnetic behaviours. The grid-type layers in these compounds are composed of octahedral metal ions bridged by carboxylate groups of 8-qoac

 Please wait while we load your content...
Please wait while we load your content...